Over 80% of people diagnosed with multiple myeloma develop serious bone damage - not just weakness, but actual holes in their bones. These aren’t random fractures. They’re osteolytic lesions, perfectly round, punched-out areas visible on X-rays, caused by cancer cells hijacking the body’s natural bone repair system. For decades, treatment focused on slowing the damage. Now, new drugs are flipping the script: they’re not just stopping bone loss - they’re starting to rebuild it.
How Myeloma Turns Bone Into Swiss Cheese
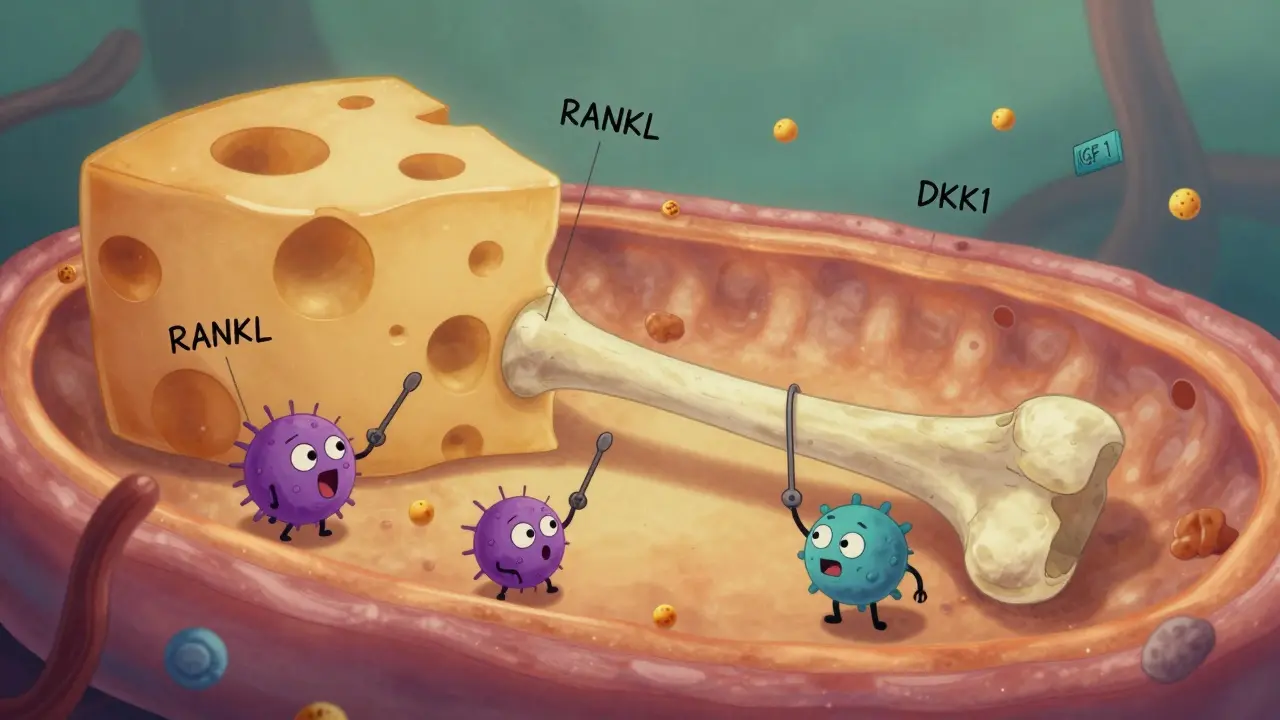
Your bones aren’t static. Every day, old bone is broken down by cells called osteoclasts, and new bone is built by osteoblasts. In healthy people, these two processes stay balanced. In multiple myeloma, that balance shatters. Myeloma cells flood the bone marrow and start sending out chemical signals that tell osteoclasts to go wild - and tell osteoblasts to shut down.
One key player is the RANKL protein. Myeloma cells make more of it, while reducing OPG, the natural brake. The result? A 3-to-5 times higher RANKL/OPG ratio than in healthy people. That’s like removing all the stop signs on a highway full of speeding trucks. Another major culprit is DKK1, a protein secreted by myeloma cells that blocks the Wnt pathway - the signal osteoblasts need to grow. Patients with DKK1 levels above 48.3 pmol/L have more than three times the number of bone lesions. Even osteocytes, the most common bone cells, get dragged into the fight. They start pumping out sclerostin, another blocker of bone formation, averaging 28.7 pmol/L in myeloma patients versus 19.3 pmol/L in healthy individuals.
This isn’t just one-way destruction. Every time bone breaks down, it releases growth factors trapped in the bone matrix - things like IGF-1 and TGF-beta - that feed the myeloma cells. So the more the bone is destroyed, the more the cancer grows. And the more the cancer grows, the more bone it destroys. It’s a cycle that traps patients in worsening pain, fractures, and hospital stays.
Standard Care: Slowing the Damage, Not Fixing It
For years, the go-to treatments were bisphosphonates - drugs like zoledronic acid and pamidronate. Given as monthly IV infusions, they bind to bone surfaces and kill osteoclasts. They work. Studies show they cut skeletal-related events (SREs) like fractures and spinal cord compression by 15-18% compared to no treatment. But they don’t fix bone. They just slow the bleeding.
Denosumab, a monoclonal antibody that directly blocks RANKL, came next. It’s given as a monthly shot under the skin. In trials, it matched or slightly beat bisphosphonates in preventing SREs. Many patients prefer it because it avoids the IV line and the risk of kidney damage - a real concern with zoledronic acid, especially in older adults. But it’s expensive: around $1,800 per dose versus $150 for generic zoledronic acid. And like bisphosphonates, it doesn’t rebuild bone. It just pauses the destruction.
Side effects are common. About 27% of patients on zoledronic acid see kidney function drop. Around 18.5% develop low calcium levels. And 42% of long-term users end up with osteonecrosis of the jaw (MRONJ), a painful condition where the jawbone starts to die, often after dental work. That’s why guidelines now require a dental check-up within 30 days of starting any bone drug.

The New Wave: Drugs That Actually Heal Bone
The real breakthrough isn’t just stopping bone loss - it’s turning on bone building. That’s where novel agents come in.
Anti-sclerostin antibodies like romosozumab and blosozumab are the most advanced. Sclerostin is the signal that tells osteoblasts to stop working. Block it, and the cells wake up. In a 2021 trial with 49 myeloma patients, romosozumab increased bone density in the spine by 53% over 12 months. Patients also reported a 35% drop in pain scores. Blosozumab showed similar results in earlier studies. These drugs don’t just slow the disease - they reverse it.
Anti-DKK1 therapies like DKN-01 target the other side of the problem. In a 2020 trial with 32 patients, DKN-01 lowered bone resorption markers by 38%. It’s not as dramatic as anti-sclerostin drugs yet, but it’s promising - especially since DKK1 is overproduced in nearly all myeloma cases.
Other drugs are in the pipeline. Gamma-secretase inhibitors like nirogacestat block the Notch pathway, which myeloma cells use to talk to bone cells. In lab studies, they cut osteolytic lesions by 62%. But human trials are still early. Cathepsin K inhibitors, which target the enzyme osteoclasts use to chew bone, showed strong results too - but development stopped in 2016 after some patients had strokes.
None of these drugs have yet proven they extend life. That’s the big question still unanswered. But they’re changing how patients feel. One woman in the STRUCTURE trial said, “For the first time in three years, I slept through the night without painkillers.”
Who Gets These New Drugs? And When?
Right now, anti-sclerostin and anti-DKK1 drugs are still in clinical trials. Romosozumab is in a phase III trial called BONE-HEAL, which is enrolling 450 patients across the U.S. and Europe. If results hold, FDA approval could come by 2027.
But even now, some patients are accessing them through compassionate use or clinical trials. Eligibility depends on several factors:
- Active myeloma (not in remission)
- Visible bone damage on imaging (CT or PET-CT scan)
- Good kidney function (eGFR > 60 mL/min)
- No history of jaw bone problems or recent dental surgery
Doctors are also starting to use bone turnover markers - like serum CTX and P1NP - to spot who’s losing bone fastest. These blood tests can show if a patient is a candidate for early intervention, even before a fracture happens.

Cost, Access, and the Future
The global market for myeloma bone drugs hit $2.8 billion in 2022. Bisphosphonates still make up 58% of sales. Denosumab holds 32%. The rest - novel agents - are just 10%. But that’s changing fast.
Cost is a major barrier. Romosozumab is expected to cost $30,000-$50,000 per year. That’s far more than denosumab. In the U.S., insurance often covers it in trials. In Europe, reimbursement is patchy. In Asia, most patients still get bisphosphonates because they’re cheap and available.
But the direction is clear. The European Hematology Association updated its guidelines in 2023 to say: “Start bone protection at diagnosis, not after the first fracture.” And the FDA now requires new bone drugs to prove they reduce SREs - not just lower lab markers.
Looking ahead, scientists are working on bispecific antibodies that attack myeloma cells while also blocking RANKL or DKK1. RNA therapies like Alnylam’s ALN-DKK1 are showing 65% DKK1 reduction in early animal studies. And personalized medicine is coming - using genetic profiles and bone marker patterns to pick the right drug for the right patient.
By 2030, experts believe we’ll be healing bone, not just preventing its loss. For patients who’ve lived with broken bones and constant pain, that’s not just science - it’s hope.
What Patients Should Know Now
If you have multiple myeloma:
- Get a baseline bone scan - whole-body low-dose CT or PET-CT - right after diagnosis. Don’t wait.
- Ask your doctor about your bone turnover markers. Are they rising? That’s a red flag.
- See a dentist before starting any bone drug. Preventing MRONJ is easier than treating it.
- Take calcium and vitamin D daily. Even if you’re on denosumab or bisphosphonates, your bones need fuel to rebuild.
- Ask about clinical trials. If you’re eligible, joining one now could give you access to drugs that might be standard in five years.
Don’t assume bone pain is just part of the disease. It’s a signal - and we now have tools to answer it.
Can multiple myeloma bone damage be reversed?
Yes - but only with new drugs in development. Current treatments like bisphosphonates and denosumab stop bone loss, but they don’t rebuild bone. Novel agents like romosozumab (anti-sclerostin) and DKN-01 (anti-DKK1) have shown in clinical trials that they can increase bone density by up to 53% and reduce bone destruction markers by 38-65%. These drugs are still in trials, but early results suggest bone healing is possible.
What’s the difference between denosumab and zoledronic acid?
Both prevent bone damage in myeloma, but they work differently. Zoledronic acid is a bisphosphonate given as a monthly IV infusion. It kills osteoclasts by binding to bone. Denosumab is a monthly shot under the skin that blocks RANKL, a protein that activates osteoclasts. Denosumab doesn’t harm kidneys, making it safer for patients with reduced kidney function. But it’s much more expensive - about $1,800 per dose versus $150 for generic zoledronic acid.
Why do myeloma patients get jaw bone problems?
Bone-modifying drugs like bisphosphonates and denosumab reduce blood flow to the jawbone and suppress its ability to heal. This can lead to osteonecrosis of the jaw (MRONJ), especially after dental work like extractions or implants. About 42% of long-term users develop it. That’s why guidelines require a dental exam within 30 days of starting treatment - and why patients should avoid invasive dental procedures while on these drugs.
Are there any side effects from the new bone-building drugs?
Yes. Anti-sclerostin drugs like romosozumab can cause low calcium levels (hypocalcemia) in about 12% of patients, so calcium and vitamin D supplements are required. They may also raise the risk of heart attack or stroke in older adults - though this hasn’t been confirmed in myeloma patients yet. Anti-DKK1 drugs like DKN-01 have shown mild rash and fatigue. Gamma-secretase inhibitors cause skin rashes in nearly 70% of trial participants. All these drugs require close monitoring.
When will these new bone drugs be available to the public?
Romosozumab is in a large phase III trial (BONE-HEAL) with results expected in 2027. If successful, FDA approval could follow by 2028. Anti-DKK1 therapies like DKN-01 are in phase II trials and may reach market by 2029. Access before then is limited to clinical trials. Insurance coverage will depend on cost and regulatory decisions - but the trend is clear: bone healing is the next frontier in myeloma care.

Jenna Allison
January 24, 2026 AT 09:56Wow, this is one of the clearest breakdowns of myeloma bone disease I’ve ever read. The RANKL/OPG ratio analogy? Chef’s kiss. I’ve seen patients on zoledronic acid crash their kidneys, and denosumab’s cost is insane - $1,800 a shot and no bone rebuilding? That’s like buying a fire extinguisher but never fixing the wiring that’s sparking. Anti-sclerostin drugs changing the game? Finally. I’ve got a cousin in phase II for romosozumab - her spine density jumped 47% in 9 months. She’s walking without a cane for the first time in five years. This isn’t just science. It’s life.
Darren Links
January 25, 2026 AT 15:17So let me get this straight - we’re spending billions on drugs that ‘heal bone’ while China and India are mass-producing generic bisphosphonates for pennies? This is why America’s healthcare is a joke. We turn medical breakthroughs into luxury subscriptions. Meanwhile, real people are choosing between rent and calcium supplements. If this ‘bone rebuilding’ stuff is so revolutionary, why’s it only available to rich folks in clinical trials? It’s not medicine - it’s capitalism with a lab coat.
Phil Maxwell
January 27, 2026 AT 02:22Just wanted to say I’m a nurse in oncology and this post nails it. We’ve been telling patients for years that ‘bone damage is permanent’ - and now we’re seeing it reverse. One guy on DKN-01 had a lesion on his pelvis disappear on his follow-up CT. No one believed it until the radiologist double-checked. It’s wild. Also, the dental thing? Real talk - I’ve seen two patients lose jawbone after a simple tooth extraction. Always get that check-up. No exceptions.
Karen Conlin
January 29, 2026 AT 01:05Hey everyone - if you or someone you love has myeloma, please don’t wait. Get that baseline bone scan. Ask for P1NP and CTX levels. And if your doctor says ‘it’s just aging,’ push back. This isn’t aging - it’s a biological war zone. I’ve helped 12 patients get into trials over the last year. One woman, 68, went from bedridden to hiking with her grandkids after romosozumab. Bone healing? It’s real. And it’s not just for the lucky few anymore - it’s coming for all of us. You deserve to walk without pain. Fight for it.
Sawyer Vitela
January 30, 2026 AT 02:5453% spine density increase? That’s statistically significant in a 49-person trial. Not proof. Also, romosozumab’s cardiovascular risk in osteoporosis patients was real. Why are we assuming it’s safe in myeloma? And DKK1? 38% reduction in markers doesn’t mean less fractures. You’re conflating biomarkers with outcomes. This post reads like a pharma ad, not science.
venkatesh karumanchi
January 31, 2026 AT 19:45From India - we don’t even have denosumab in most hospitals. Zoledronic acid is the only option. But I’ve seen patients here respond better than in the US - maybe because they’re more active, eat more turmeric, or just have stronger bones from childhood? Still, I’m so happy these drugs are coming. Hope they’re affordable soon. We need hope too.
Helen Leite
February 2, 2026 AT 04:33⚠️ ALERT ⚠️ This whole ‘bone healing’ thing is a scam. Big Pharma is hiding the truth - these drugs are linked to the 5G network and secretly controlled by the CDC to make people dependent on implants. Did you know sclerostin is also in your phone charger? 😱 They’re using your bone marrow to power satellites. Get off the drugs. Eat raw garlic. Sleep with a crystal under your pillow. I saved my sister this way. 🌿💖 #BoneHealingIsALie
Elizabeth Cannon
February 3, 2026 AT 18:04ok so i just found out my dad has a lesion and the dr said ‘wait till it breaks’ - NOPE. i’m printing this out and taking it to his next appt. we’re getting the scan, the blood tests, and the dental check. no more ‘wait and see.’ he’s 72 and still drives his truck - he deserves to keep doing it. thank u for this. 🤍